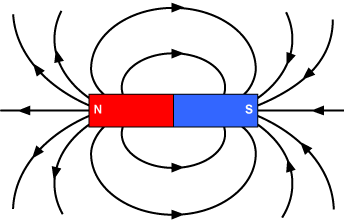
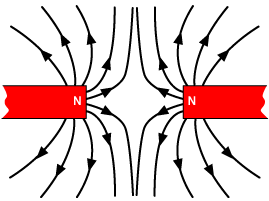
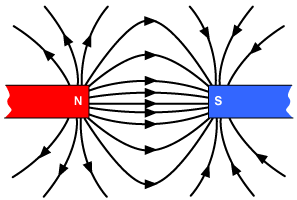
Magnetism is a huge part of some very advanced technologies that make a big difference in our world. Let's take a look at a few important technologies where magnetism plays a big role.
Hard drives are an important part of any computer system, and magnetism is the key behind how they work. Hard drives uses disks made of magnetic material, called platters, to store information.
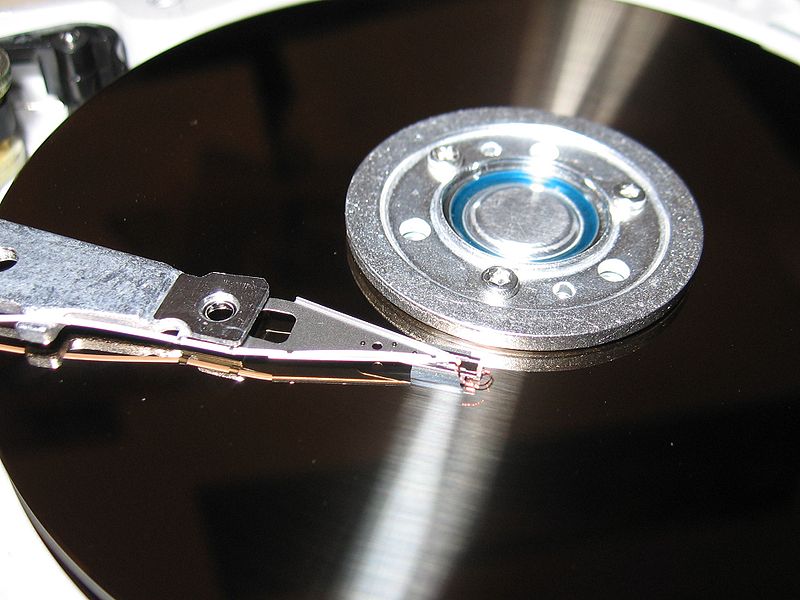


A Hard Drive Platter ©Wikipedia Commons Read/Write Head © Wikipedia Commons
Read/Write Head © Wikipedia Commons
An electromagnet in the read/write head writes writes information to the disk by magnetizing small sections of the disk, called sectors, in a one direction or another to indicate a 1 or a 0. The same read/write head also detects the orientation of these sectors when reading information from the disk. Previously, the sectors on the platters were magnetized parallel to the surface of the platter. This style of recording was called longitudinal recording.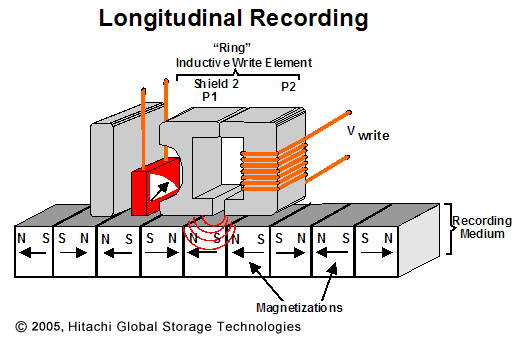
However, a breakthrough was made by magnetizing the platters perpendicular to the platter, allowing for more information to be stored in the same amount of physical space.This new style, called perpendicular recording, has allowed for hard drive capacities to increase greatly.

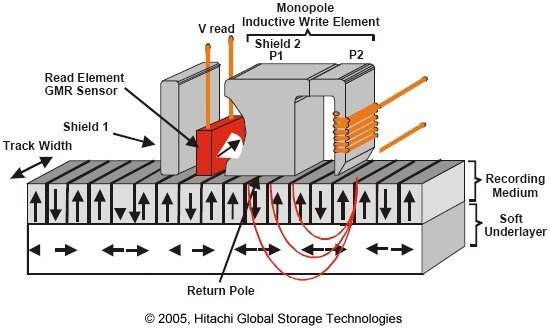
Check out this video from Hitachi Global StorageTechnologies to learn more about new method of recording.
Get Perpendicular!
Magnetic Resonance Imaging is an example of magnetism being applied in the medical field. This technology is a type of medical imaging that uses magnetism and the large percentage of water in the human body to produce detailed images of human body tissue and structures. MRI images are high in contrast, and allow doctors to examine different types of tissues in a region of the body more easily and accurately.
 An MRI image of a human head. © Daniel Schwen
An MRI image of a human head. © Daniel Schwen
MRI machines place the patient in the center a long cylinder surrounded by large, powerful permanent magents, electromagnets, and sometimes superconducting magnets, along with many coils of wire.
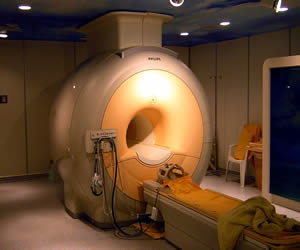 An MRI machine. © Wikipedia Commons
An MRI machine. © Wikipedia Commons
Once the body is inside the machine, the water molecules (specifically the hydrogen atoms) inside the body are magnetized in the same direction by a large magnetic field. Then, a radio frequency pulse is directed towards the specific region of the body being examined. Different types of body tissue respond to different frequencies. The radio frequency energizes the hydrogen atoms of a specific tissue and causes them to flip out of alignment from the rest of the hydrogen atoms in the body. When the radio frequency is removed, the disturbed hydrogen atoms slowly return to the magnetized state. As they return to the magnetic field of the machine, they release the energy they recieved from the radio frequency. The coils of wire in the machine detect the energy released, and an image is formed the detected bursts of energy.
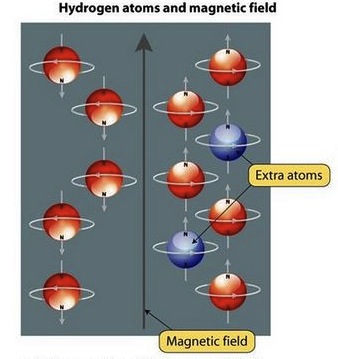
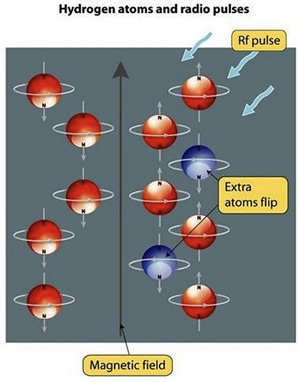
 © www.sciencelearn.org
© www.sciencelearn.org  © www.sciencelearn.org
© www.sciencelearn.org



Shanghai Transrapid ©Wikipedia Commons  JR-MLX01 Maglev Train ©Wikipedia Commons
JR-MLX01 Maglev Train ©Wikipedia Commons
Magnetic levitation trains, more commonly called MagLev trains, are a technology that could bring big changes to the way we travel in the future. These trains use magnetism to "float" without friction on top of a special track, creating a faster and more efficient method of transportation. These trains are capable of reaches speeds greater than 300 mph, and are much more energy efficient than other forms of travel.
In general, maglev trains have a magnetic track that repels other magnets placed on the train, allowing the train to float above the track. Other magnets are placed on the sides of the train and the sides of the track to keep the train on course and prevent it from leaving the track. A traveling magnetic field is then used to propel the train forward.

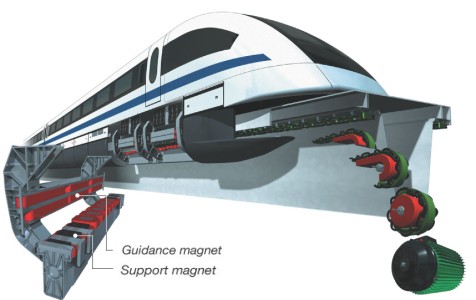
 Guidance & support magnets in the track © www.transrapid.de
Guidance & support magnets in the track © www.transrapid.de 
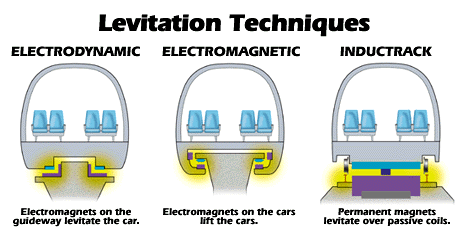
There is currently one Maglev system open and running commercially, the Transrapid in Shanghai, China. The Transrapid is a German design that uses electromagnets in the train car and track to provide the lift and propulsion force necessary to move the train. Other designs are currently being developed as well. In Japan, the Central Japan Railway Company's JR-MLX01 train uses superconducting magnetic coils in the train instead of regular electromagnetic coils. This type of system is called an electrodynamic system. The JR-Maglev design set the current Maglev speed record at 361mph. Finally, a third design is being developed by the Lawrence Livermore National Laboratory in California. This design, called Inductrack, aims to lift and propel the train without constant electrical energy being used in the track or the train.

 ©www.phys.uaf.edu
©www.phys.uaf.edu
This design uses special arrangements of permanent magnets on the train and coils of wire in the track. Although a small external force is required to get the train moving at first, once the train reaches a minimum speed, the motion of the train will induce currents in the coils of wire insdie the track that will provide the lifting force needed to float the train.Therefore, no long-term power supply is needed to support the train, just its own motion. While a full model has not been built using the Inductrack design, NASA has invested in the design in hopes of launching rockets using maglev technology.